GenAI-enabled Global Value Dossiers
Navigating market access requires compelling, evidence-rich Global Value Dossiers (GVDs) that are accurate, adaptive, and aligned with payer expectations. MadeAi-GVD streamlines the end-to-end development of both traditional and interactive GVDs—leveraging GenAI and domain expertise to reduce manual burden, accelerate timelines, and ensure local adaptability.
Whether you're preparing for HTA submissions, AMCP dossiers, or global affiliate support, MadeAi offers an intelligent, transparent solution for building modular, customizable, and audit-ready dossiers at scale.
GenAI-enabled Global Value Dossiers
Navigating market access requires compelling, evidence-rich Global Value Dossiers (GVDs) that are accurate, adaptive, and aligned with payer expectations. MadeAi-GVD streamlines the end-to-end development of both traditional and interactive GVDs—leveraging GenAI and domain expertise to reduce manual burden, accelerate timelines, and ensure local adaptability.
Whether you're preparing for HTA submissions, AMCP dossiers, or global affiliate support, MadeAi offers an intelligent, transparent solution for building modular, customizable, and audit-ready dossiers at scale.
Lorem ipsum dummy text
Why Choose MadeAi for GVD?
- Up to 50% faster GVD creation cycles
- Full transparency and source traceability
- Scalable support for multiple therapeutic areas
- Built-in alignment with QALY, RWE, and HTA standards
- Proven by top-15 pharma and global biotech innovators
Why Choose MadeAi for GVD?
- Up to 50% faster GVD creation cycles
- Full transparency and source traceability
- Scalable support for multiple therapeutic areas
- Built-in alignment with QALY, RWE, and HTA standards
- Proven by top-15 pharma and global biotech innovators

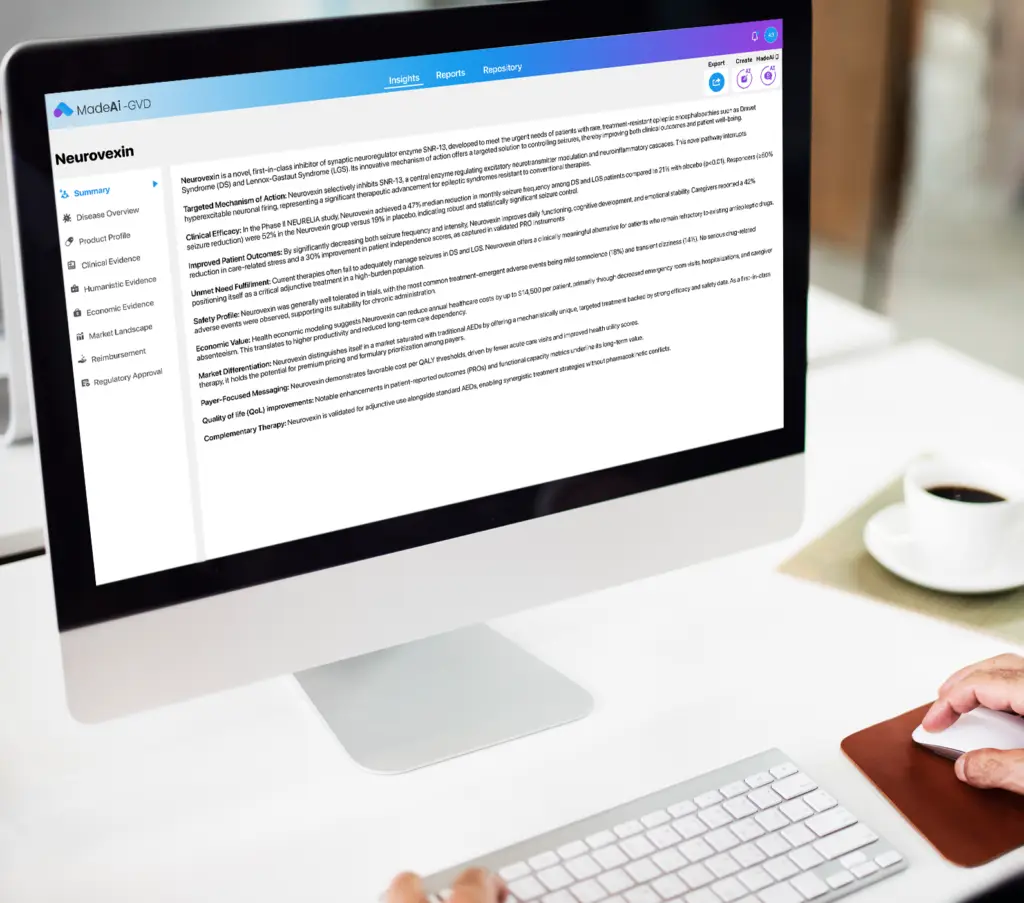
What MadeAi Delivers for GVDs
MadeAi streamlines Global Value Dossier development by accelerating evidence synthesis, ensuring full source traceability, and aligning with HTA, QALY, and RWE standards. Its GenAI-powered platform supports multiple therapeutic areas with scalable precision, reducing GVD creation cycles by up to 50%. Built for compliance and speed, MadeAi is trusted by leading pharma and biotech teams to deliver audit-ready, insight-rich dossiers at scale.
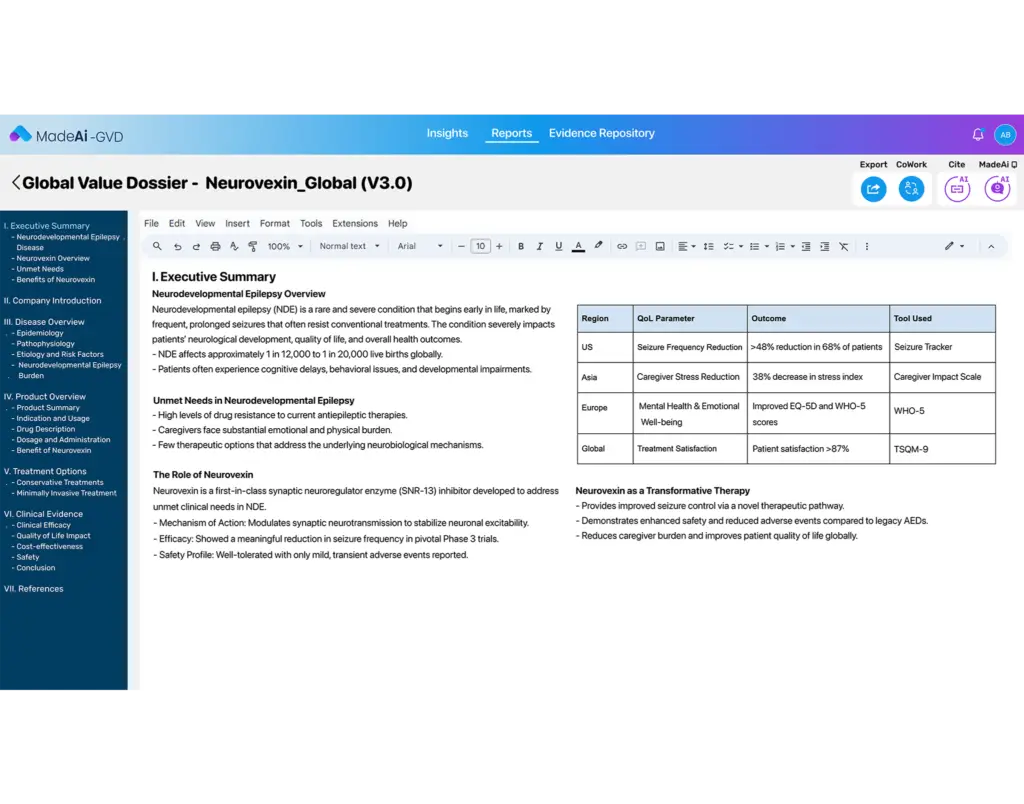
End-to-End Dossier Development
From disease burden analysis to modeling and market access strategy, MadeAi helps teams assemble comprehensive and compliant dossiers with speed and precision.
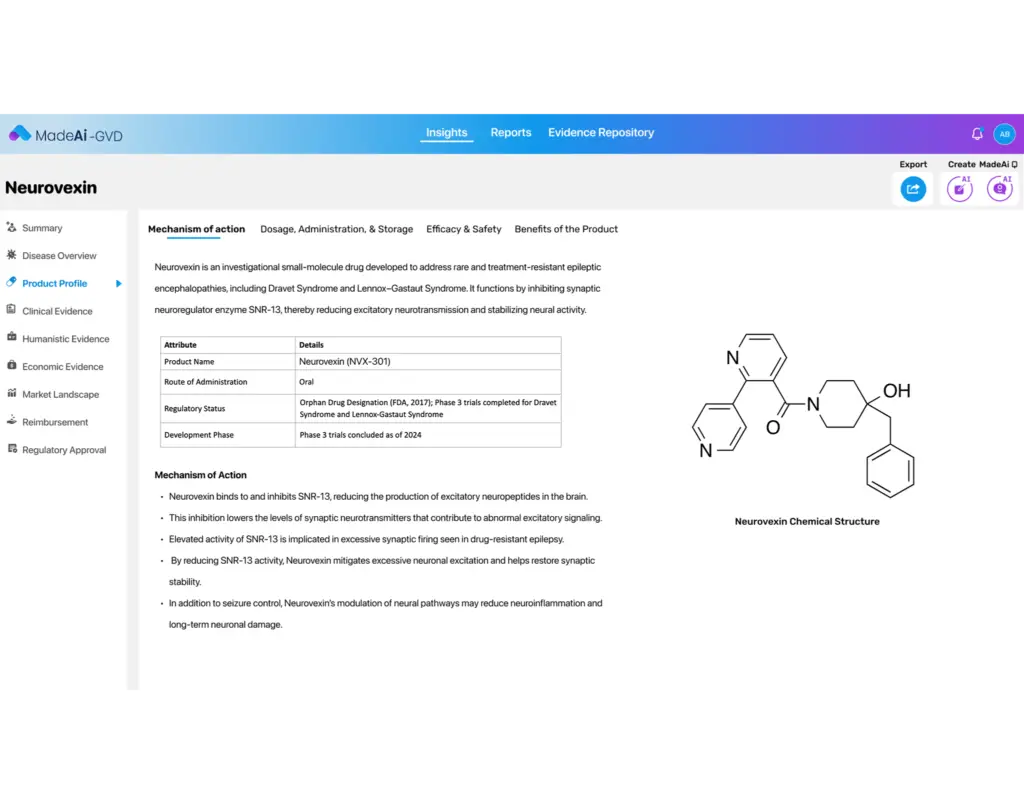
AI-Assisted Content Generation
Automatically draft core sections like value stories, unmet need narratives, and product positioning using AI trained on medical, regulatory, and payer-relevant content.
Interactive GVD (iGVD) Options
Support for dynamic, stakeholder-specific digital dossiers that include visualization tools, slide generators, and adaptive navigation for cross-functional use.


Integrated Compliance Frameworks
Built-in checks and references for HTA and AMCP standards to ensure submission-ready outputs and local market adaptation needs.
Collaboration and Version Control
Assign roles to medical, HEOR, regulatory, marketing, and legal teams with centralized input tracking, version history, and modular content updates.
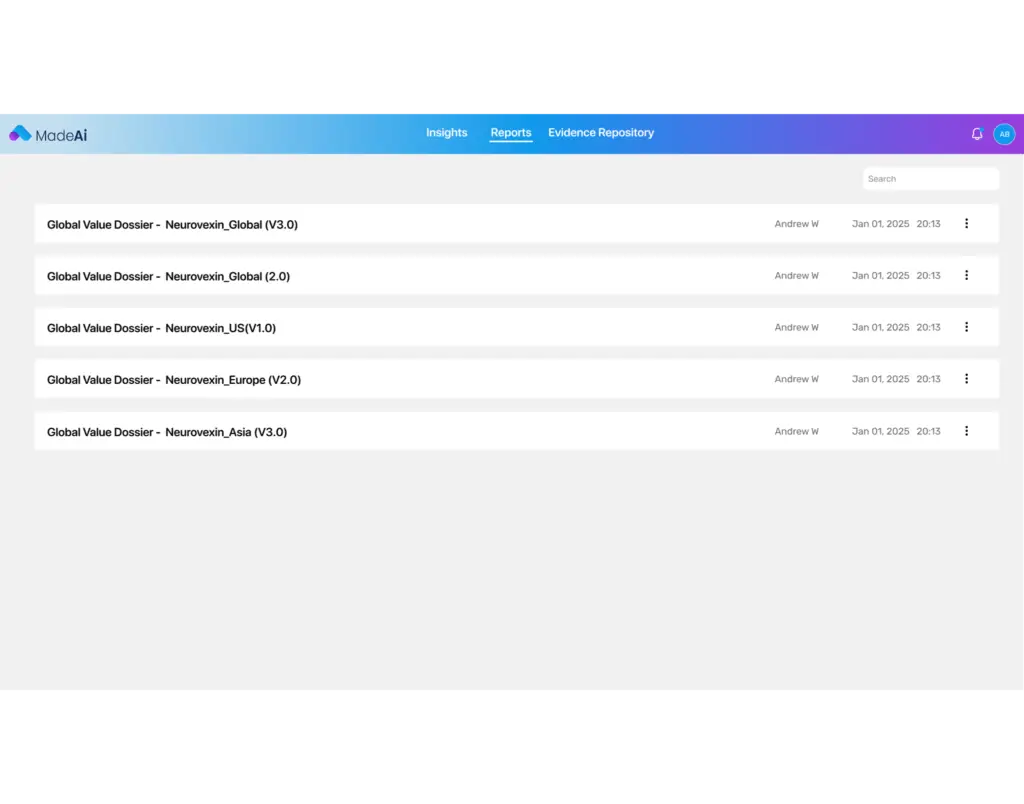

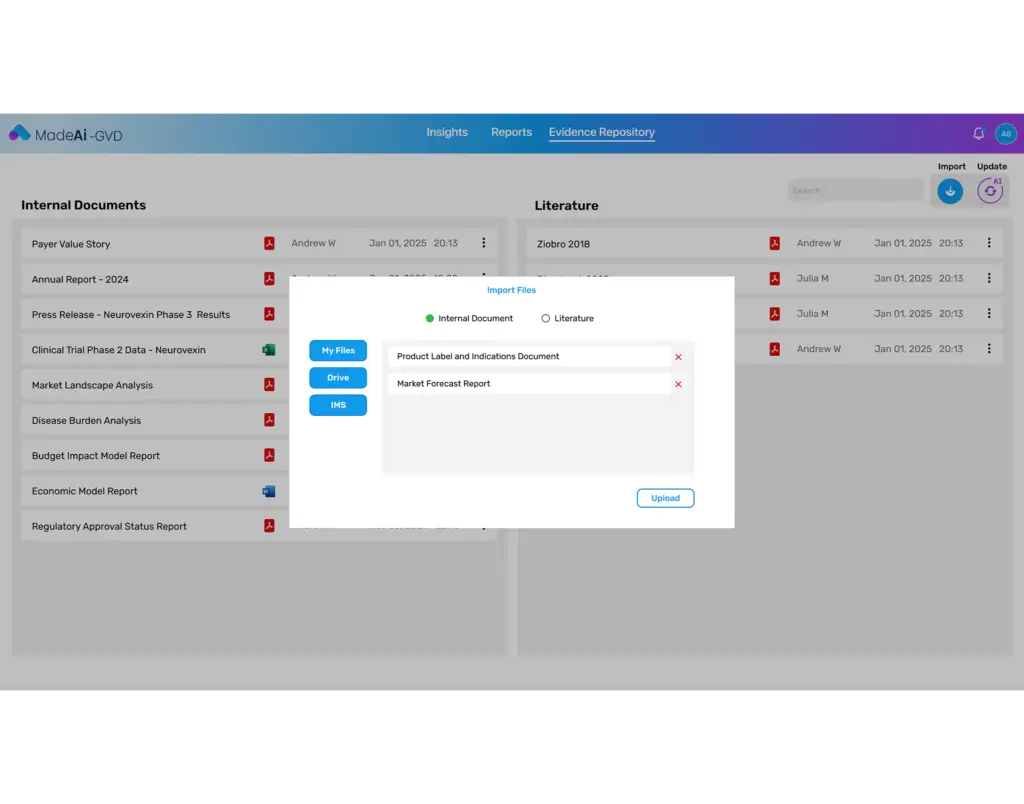
Searchability & Access
With MadeAi’s GenAI-enabled InsightsBot, information with in GVDs becomes simple search and access, empowering teams to find critical data and documents quickly freeing up resource time.
Living GVD
The emergence of new clinical data, regulatory changes, and market access updates are easily managed with MadeAi’s Living GVD, which automatically monitors for and incorporates relevant market events to keep GVD’s up to date.
Who It’s For
- HEOR & Market Access Teams:
Deliver high-quality dossiers across global markets - Regulatory & Compliance Teams:
Ensure traceability and alignment with agency requirements - Medical Affairs:
Communicate differentiated clinical value clearly and efficiently - Launch Teams:
Scale dossier creation across multiple product launches


Contact Info
Connect with a MadeAi representative to see how we can support your GVD development needs.


